Research
Curavista.Health is innovative and cutting edge; it secondary use of data is built into the platform by default.
Proven successful in more than 50 (inter)national trials.


Consent (IC)
- Complies with the legal obligations.
- Includes on-screen signature saved as PDF.
Smart forms
Smart forms provide:
- Inclusion or exclusion based on responses.
- assigning the appropriate study arm
- start follow-up forms and reminders of the right study arm.
If excluded, the study stops for this person immediately.
Including can be done by a professional either by approaching people directly online.

Unique individuals
- Each user is unique with their own profile.
- The module and language are "latched" to the profile.
- The app automatically displays correct language and modules.
- Each user follows their own track within the study.
International trials are easy to coordinate from one location. coordinated.
Create your own app for your studies.
Available Languages: Arabic, Danish, German, English, French, Greek, Italian, Ukrainian, Dutch, Norwegian, Polish, Portuguese, Spanish, Turkish.
Data sources
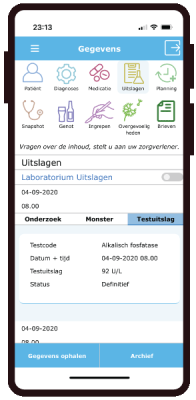
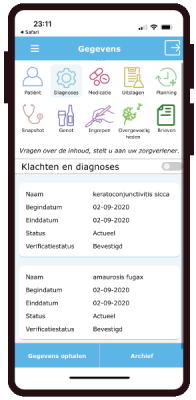
eCRF
- Forms with manual entry as eCRF, diary or PROMS.
- Flexible and smart;
- if.... then...
- min-max value
- calculations,
- fill-in rolls,
- reminders
- alerts
- start next track etc.
- 200 PROMS available
- Data extraction as CSV or excell with codebook.
Big Data
- via PBM all medical data in structured data format
- via connected devices;
- Findair smarthaler
- Fitbit activity tracker
- Omron blood pressure monitor
- Omron scale
- Spirobank Smart
- Spirobank Smart Oxi
Curavista.health retrieves and archives the data in a structured manner.
Extractions
Oracle database
Technical
Learn more about our cloud-based toolbox, layered architecture, development process or what software we use.
Audits
Curavista and its platform are certified.
Additional third-party audits are possible if required.
CE certification
The platform is one engine, CE certified. Each module follows the same configuration steps and thus fits the CE mark. Although studies usually do not require this quality mark, it is relevant for the period after a study. The module can then be offered to the general public.

Certifications
Curavista.health is a certified medical device. So you can count on a safe and reliable system.
Own module
The main advantages of an "Own Module" at a glance:
- Proprietary content;
- Tailored Informed Consent;
- Choose languages and countries;
- A customised dashboard;
- Proprietary data extractions;
- Exclusive governance.
Economies of scale
The platform is one engine, controlling all modules. This offers advantages:
- configuration of software reduces the risks involved in regular software development by 90%;
- shared cost of certifications;
- management and operations are centralised.
- helpdesk for technical support